Building out Site Research Capabilities: A Barrington James Perspective
21 Jul, 202515 minsThe clinical research industry is experiencing a pivotal shift. Increasingly complex protoco...

The clinical research industry is experiencing a pivotal shift. Increasingly complex protocols heightened regulatory scrutiny, and demands for faster therapeutic development are pushing research operations to evolve. For single-site clinical trial organizations, this changing landscape offers both opportunity and risk. The opportunity lies in scaling operations into a multi-site network and ultimately transforming into a Site Management Organization (SMO). The risk stems from navigating this transformation without a structured approach. This paper lays out a clear and actionable blueprint, incorporating strategic frameworks, operational best practices, and the latest thinking in research infrastructure to guide organizations through this journey.
Introduction
Transitioning from a single clinical site to a robust multi-site network and eventually to a full-fledged SMO is a substantial undertaking. It requires a fundamental shift in how an organization operates, manages people, and leverages technology. This transformation is not merely about adding more locations but winning over principal investigators (PIs) and reimagining the operational model to ensure scalability, compliance, and quality. Table 1 provides a high-level overview of how to frame the distinctions across each stage of growth. An expanded, more detailed table may be found in Appendix A.
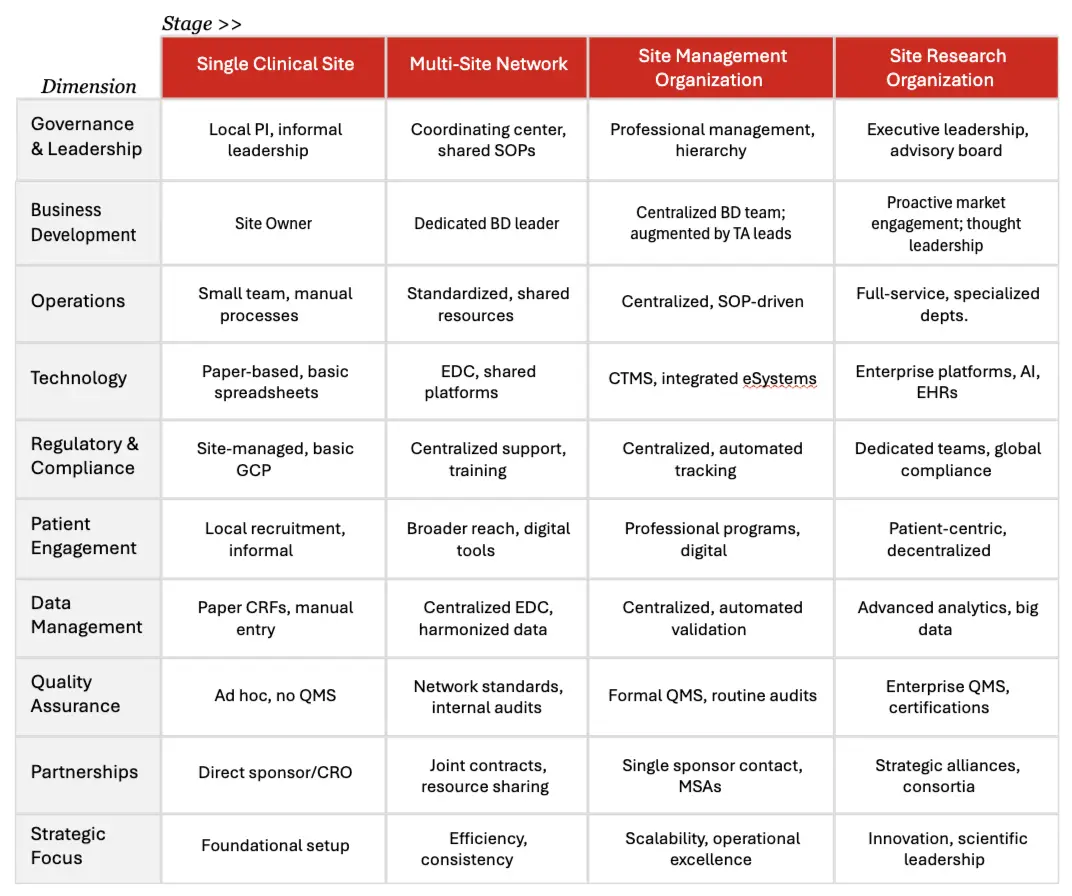
Table 1. General Operations By Stage
A holistic approach is essential, encompassing leadership development, standardized procedures, centralized services, and cutting-edge technology solutions. This white paper offers a phased, structured methodology grounded in industry best practices and strategic insights.
The Case for Expansion
The rationale for expanding beyond a single site is compelling. As patient access becomes more nuanced, driven by advancements in more personalized, novel therapies, sponsors and CROs increasingly prefer working with partners that demonstrate operational maturity, scalability, and reliable performance across geographies. Expanding into a site network not only facilitates access to a more diverse patient population but also enhances the organization's appeal to sponsors seeking dependable recruitment and data quality.
Beyond competitive fitness, strategic benefits include economies of scale in staffing and operations, increased negotiating leverage, and more consistent data quality through standardized processes. Organizations that successfully transition to multi-site operations often realize improved contract terms, faster study start-up times, and stronger reputational standing within the research ecosystem.
However, a site’s growth agenda must first and foremost focus on winning over PIs in an increasingly competitive market.
Sponsors and research organizations compete for a limited pool of experienced PIs, often relying on a finite number of KOLs (key opinion leaders) who have made significant contributions in advancing their respective fields via practice, research and publications. And even while the demand for clinical research has persisted, it is estimated that only 4% of practising physicians participate. In the main, this lack of interest/participation may be contributed to:
- High Turnover / "One-and-Done": About half of all PIs conduct only a single clinical trial before leaving the field, a trend known as the "one-and-done" effect. Some estimates put this churn impact as high as 54%.
- Workload and Administrative Burden: Many physicians find it difficult to balance research responsibilities with clinical practice. The time required for trial implementation, regulatory and safety reporting, and complex documentation are major deterrents.
- Financial Dissatisfaction: Compensation often does not reflect the true effort required, leading to dissatisfaction and attrition among PIs.
- Increasing Complexity of Trials: Modern clinical trials are more complex, requiring more oversight, documentation, and adherence to protocols, which further discourages participation.
- Ageing Workforce: Many experienced investigators are retiring, and fewer new investigators are entering the field, exacerbating the shortage.
Indeed, with only ~40,000 PIs and >1,000,000 practicing in the US alone (ACRP), it is no wonder Integrated Research Organizations (IROs) and other similar firms look to wrap clinical research capabilities around a physician and reduce the perceived burden of participation.
Winning Over PIs
Given this competitive landscape, sourcing an experienced PI or recruiting/upskilling treating physicians to become principal investigators (PIs) is a critical first step. This transformation begins with a thorough assessment of their motivation, alignment with therapeutic areas of interest, and access to relevant patient populations. Physicians must be genuinely interested in research and willing to dedicate the time and energy required to fulfill their regulatory and ethical obligations.
Critically, the financial opportunity must be appealing. Aside from interest in conducting clinical research, the PI must have clear line of sight to the potential incremental earnings for participating in a site/network. A key challenge for leadership will be to articulate the economics based on the planned hours required per study, and the volume of studies the PI will potentially attract.
For leadership bringing on a PI is often a chicken/egg decision… they would like to hire a PI to win studies, but they don’t want to hire a PI until they have a contract with a sponsor.
Barrington James Insight: Before hiring a PI full-time, bring on a PI contractually, based on a retainer model with an agreed-upon hourly rate once trials start to come in. Once the volume justifies a full-time offer, convert the PI contract accordingly. A sample financial model may be found here https://www.barringtonjames.com/resources/download/key-considerations-when-hiring-a-principal-investigator--pi-/
To support this transition, comprehensive training is essential. Investigating physicians must be well-versed in Good Clinical Practice (GCP), FDA regulations, ICH guidelines, and the responsibilities of an investigator, including safety reporting and investigational product oversight. Protocol-specific and SOP-based training should be supplemented with administrative education around budgeting, contracting, and patient recruitment strategies.
Equally important is establishing a strong operational support system around new PI’s. This includes hiring (or contracting) clinical research coordinators, research nurses, regulatory specialists, and administrative staff. See Appendix B for a breakdown of key roles and responsibilities. A well-rounded team ensures operational efficiency, allows the PI to focus on oversight, and supports patient recruitment and retention. Ethical considerations such as conflict-of-interest management and informed consent must also be addressed proactively to safeguard participant welfare.
Organizations should also invest in cultivating a culture of research among clinicians. Hosting educational seminars, supporting attendance at industry conferences, and offering mentorship opportunities can help increase physician engagement and enthusiasm for research. Aligning incentives, such as research-based bonuses or dedicated clinic time, may also help convert capable clinicians into committed investigators.
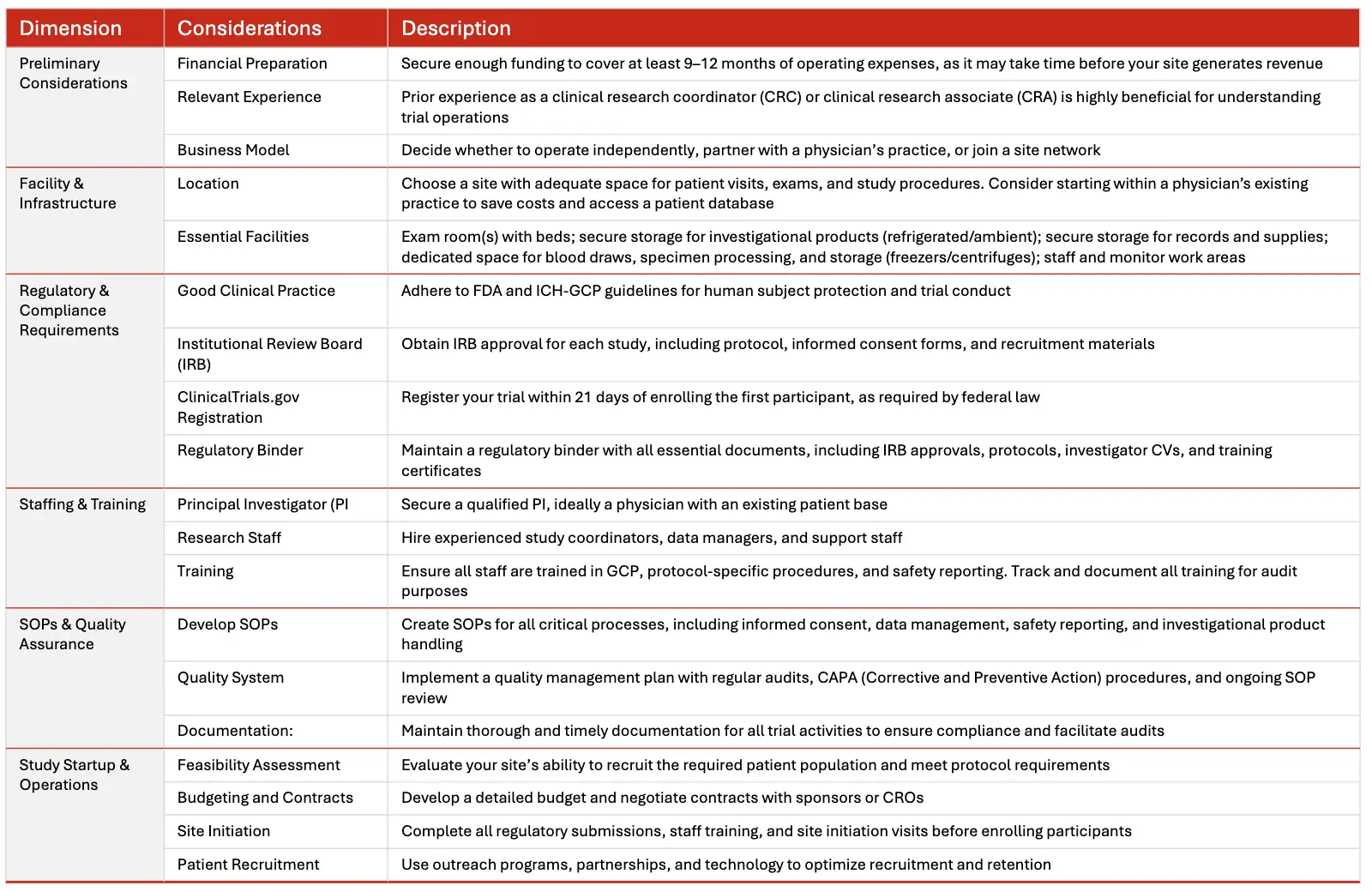
Establishing a Clinical Trial Research Site
Establishing a clinical trial research site is a significant undertaking that requires careful planning, regulatory compliance, and operational excellence. Below is a step-by-step guide and best practices to help you get started.
Scaling to a Site Network
Once a stable foundation of investigators and support staff is in place, the next phase involves replicating and scaling this model across multiple locations. Success in this phase hinges on building a capable leadership team and implementing consistent operational standards. Key leadership roles should include a Director of Site Operations, Business Development Lead, Clinical Operations Managers, and a dedicated Quality Assurance Officer.
Standardization is the cornerstone of scalable operations. Organizations must develop comprehensive SOPs that govern every aspect of trial execution, from feasibility assessments to close-out procedures. These SOPs should be centrally managed and regularly updated to ensure uniformity across sites. Centralizing services such as budgeting, contracting, regulatory submissions, and patient recruitment will further enable efficiency and consistency.
Quality assurance must be institutionalized through a formal Quality Management System (QMS). This system should facilitate internal audits, corrective action plans, and continuous improvement initiatives. Performance metrics such as enrollment rates, data query resolution times, and audit outcomes should be tracked rigorously to monitor and enhance site performance.
From a technology perspective, a centralized Clinical Trial Management System (CTMS), electronic data capture (EDC) tools, and electronic Investigator Site Files (eISF) are essential to maintain operational transparency and regulatory compliance. Centralized databases for patient recruitment and HIPAA-compliant communication platforms further support coordination across geographically dispersed sites.
During this phase, financial discipline becomes even more critical. Organizations must build scalable budgeting frameworks and develop financial dashboards that track cash flow, site performance, and profitability in real time. Strong financial oversight ensures that investments in infrastructure and personnel yield sustainable returns.
Evolving into a Site Management Organization (SMO)
The final stage of the transformation involves transitioning into a full-service SMO. At this level, the organization offers a broad suite of services to sponsors and CROs, including centralized feasibility, regulatory management, budget negotiation, patient recruitment, data quality oversight, and monitoring coordination. This evolution is marked by the ability to act as a strategic partner rather than a passive site operator.
Specialization becomes increasingly important at this stage. Focusing on specific therapeutic areas where the organization has demonstrated expertise and patient access can differentiate the SMO and make it more attractive to sponsors seeking targeted capabilities.
Strategic partnerships also become a growth catalyst. Building strong relationships with CROs, sponsors, and technology vendors allows the SMO to offer more integrated and efficient services. More forward-leaning organizations will also develop partnerships with key functional service providers to augment site capabilities with critical services such as biostatistics, labs, regulatory, etc. to serve as an end-to-end Site Research Organization (SRO).
On the commercial front, dedicated account management, therapeutic area strategists that can weigh in on protocol design and the ability to share/articulate the organization’s value proposition are critical.
Advanced technology integration is another hallmark of a mature SMO. Systems should be interconnected to provide seamless oversight across CTMS, eTMF, financial systems, and QMS platforms. The use of business intelligence tools, digital patient engagement solutions, and artificial intelligence for site selection and risk prediction further enhances efficiency and decision-making.
Compliance and risk management must also evolve. A proactive approach to legal and regulatory issues, including robust cybersecurity protocols and specialized legal counsel, is essential for long-term sustainability. SMOs should consider establishing a compliance committee and hiring a Chief Compliance Officer to lead proactive regulatory readiness initiatives.
Key Challenges and Mitigation Strategies
While expansion offers significant advantages, the journey is not without its obstacles. Fractionally deployed physicians frequently find themselves juggling clinical duties with research commitments, a challenge that can be alleviated through clear policies and robust institutional backing.
New investigators often begin with limited experience, underscoring the importance of comprehensive mentorship and a well-structured onboarding process. Ensuring operational consistency across multiple sites can be intricate, but centralized governance and regular audits can help uphold uniform standards. Successfully integrating diverse technology systems also requires careful attention, whether that means replacing outdated platforms or harmonizing them into an effective, unified network.
As organizations grow, maintaining regulatory compliance demands ongoing focus and vigilance. Strategies such as establishing a dedicated compliance team, providing continuous staff training, and implementing real-time monitoring are essential. Financial planning should be carefully staged to align with growth, supported by a mix of stable revenue streams. Attracting and retaining talented staff relies on fostering a positive culture that emphasizes career development and offers competitive compensation. Effectively guiding an organization through change depends on open communication and strong leadership alignment.
And lastly, but not of less priority, is ensuring operational resilience. This involves preparing contingency plans for protocol deviations, supply chain disruptions, and key staff turnover. Embedding these plans within the quality management system and regularly updating them through cross-functional exercises enables organizations to better anticipate and withstand potential disruption.
Conclusion and Strategic Recommendations
The path from single-site operation to Site Management Organization is transformative. It requires vision, discipline, and execution. Organizations that succeed are those that invest in strong leadership, develop standardized and centralized operations, adopt advanced technologies, and foster a culture of quality and compliance.
Strategically, the journey must begin with deliberate talent development and be supported by structured processes and systems. Technology must be viewed as an enabler rather than a challenge, and strategic partnerships should be cultivated to enhance service offerings. Financial and operational planning should be aligned to support sustainable growth.
Organizations should also engage in long-term strategic planning, including the development of a growth roadmap, an innovation strategy, and an external advisory board to ensure continual alignment with market trends and sponsor needs. By adhering to this blueprint, organizations can position themselves as reliable, high-performing research partners‚ capable of accelerating drug development, improving patient outcomes, and driving long-term business value in the clinical research ecosystem.
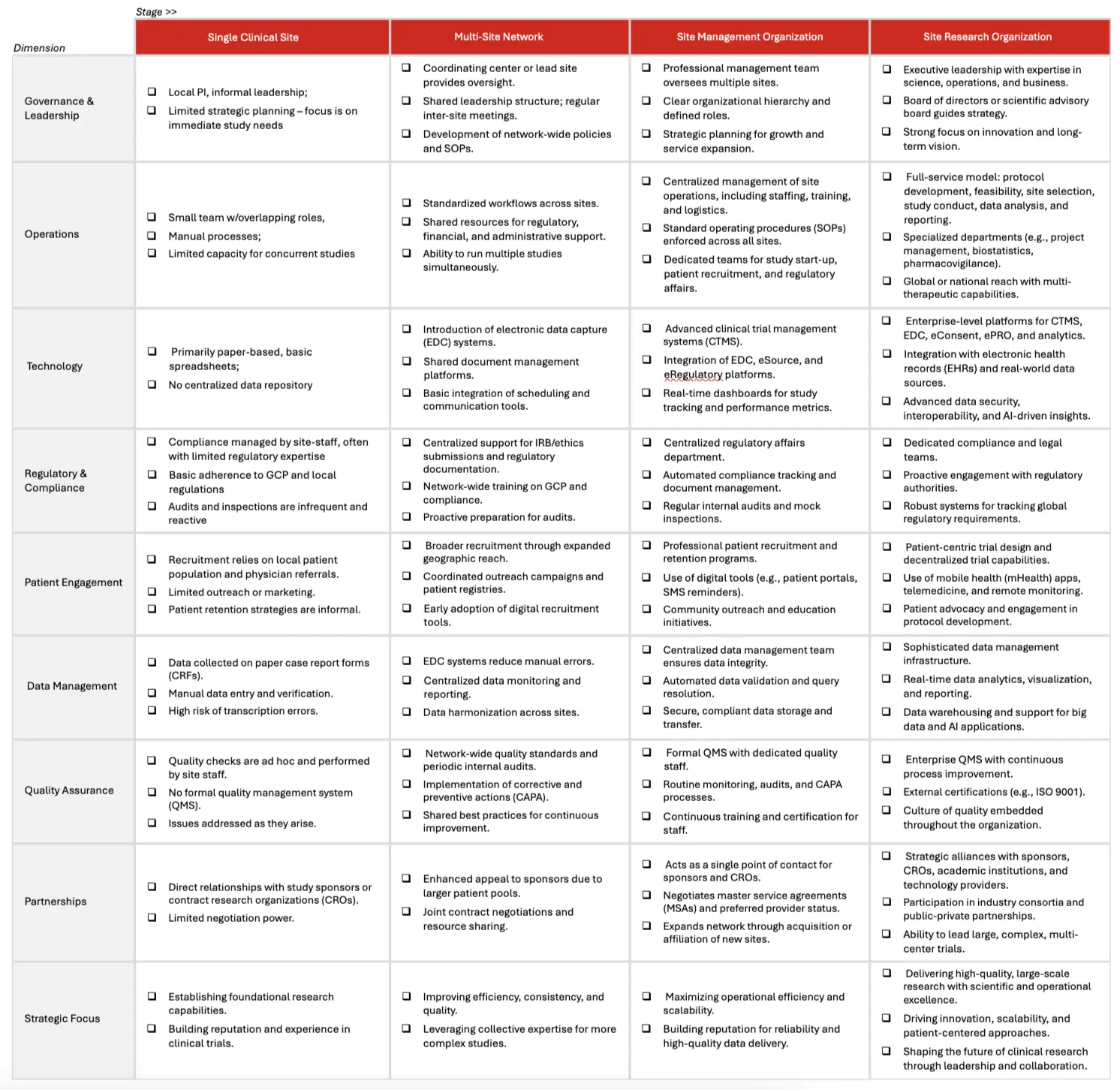
APPENDIX A: Site Research Maturity Model
This enhanced maturity model provides a comprehensive framework for the evolution of site research organizations, detailing the progression from a single clinical site to a sophisticated site research organization.
APPENDIX B: Site Augmentation Solutions
When we speak about controlling the ‘controllables’ in clinical research, clinical trial site performance, and physician/patient participation are usual near, if not at, the top of the list. As clinical trials become more complex, patient populations more nuanced, site resourcing increasingly constrained and physician clinical trial participation hovering at ~3%, there is no shortage of risk once a clinical trial is kicked off by a sponsor and it’s operational partners. Indeed, teasing out and planning for any “controllable” elements has never been as critical.
To this end, we at SSG, provide Site Augmentation Services to solve for areas that introduce the most risk for sponsors: underfunded sites, unclear patient access and operational burden.
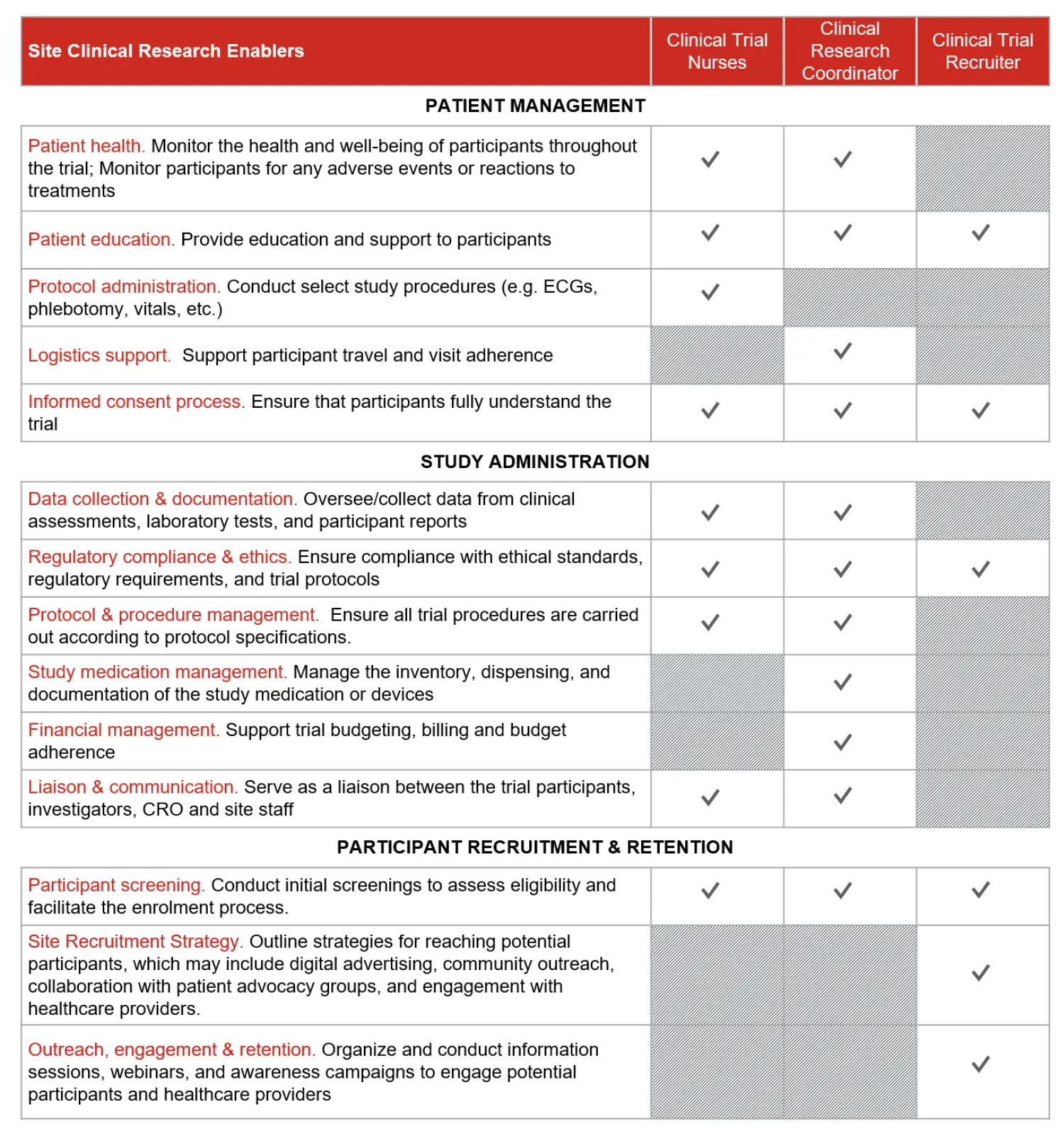
Within the context of your clinical trial, we provide Clinical Trial Nurses, Clinical Research Coordinators and Clinical Trial Recruiters to facilitate site activation, patient recruitment and study administration. Providing on-site support to investigators and study coordinators, our professionals ramp-up quickly and seamlessly integrate into partner site clinical research workflows.

Common support includes, but is not limited to:
1. Patient management
2. Study administration activities
3. Patient recruitment & retention
Team Roles & Responsibilities
Three roles ramp up quickly to focus on those enabling activities critical to overcoming challenges in site staffing, recruitment and administrative burden: clinical trial nurses, clinical research coordinators and clinical trial recruiters.
Tangible impact for patients… tangible impact for sites and sponsors
Partners who have leveraged our site augmentation services (SAS) have realized improved trial timelines through accelerated site activation, enhanced data quality, increased patient recruitment and retention rates, and trusted compliance with regulatory standards. In addition, there have been several less direct, but no less advantageous, benefits for sites and sponsors:
Access to the latest technologies: Site2 deploys proprietary advanced automation technologies to drive patient referrals by support health care provider awareness and engagement, increasing key opinion leader (KOL) community involvement and seeding a sponsor’s brand in the market.
Navigate increasingly decentralized clinical trial constructs: The growing focus on decentralized clinical trials (DCTs) presents both challenges and opportunities for site augmentation services. As trials expand from traditional, site-centric models to more patient-centered approaches, Site2Site Augmentation Services connects the dots between remote patient monitoring, virtual consultations, and electronic data capture; ensuring patient engagement and data integrity in a less controlled environment.
Trusted regulatory expertise: As regulatory bodies worldwide continue to update guidelines to accommodate the rapid pace of technological advancement and the adoption of novel trial designs, our partners benefit from the deep regulatory expertise from the broader Site2 team to stay ahead of these changes. This enables our Clinical Research Coordinators to provide expert guidance to partner sites on navigating the regulatory landscape, ensuring compliance, and adapting to new requirements. This includes understanding the implications of global regulations on data privacy, cross-border data transfer, and patient consent in an increasingly digital and interconnected world.
Bottomline –
Site augmentation services represent a vital strategy for overcoming the headwinds for sites and investigators to participate in clinical research. By providing specialized support and expertise across clinical practice, administration and patient recruitment, these services enhance the efficiency, effectiveness, and quality of trial execution, improving outcomes and overall medicinal value.
Get in Touch
To explore how Barrington James Strategic Solutions Group can become a trusted partner in your organization’s long-term success, connect directly with our leadership.
Contact:

Bryan Katz
President, Barrington James Strategic Solutions Group
BKatz@barringtonjames.com

